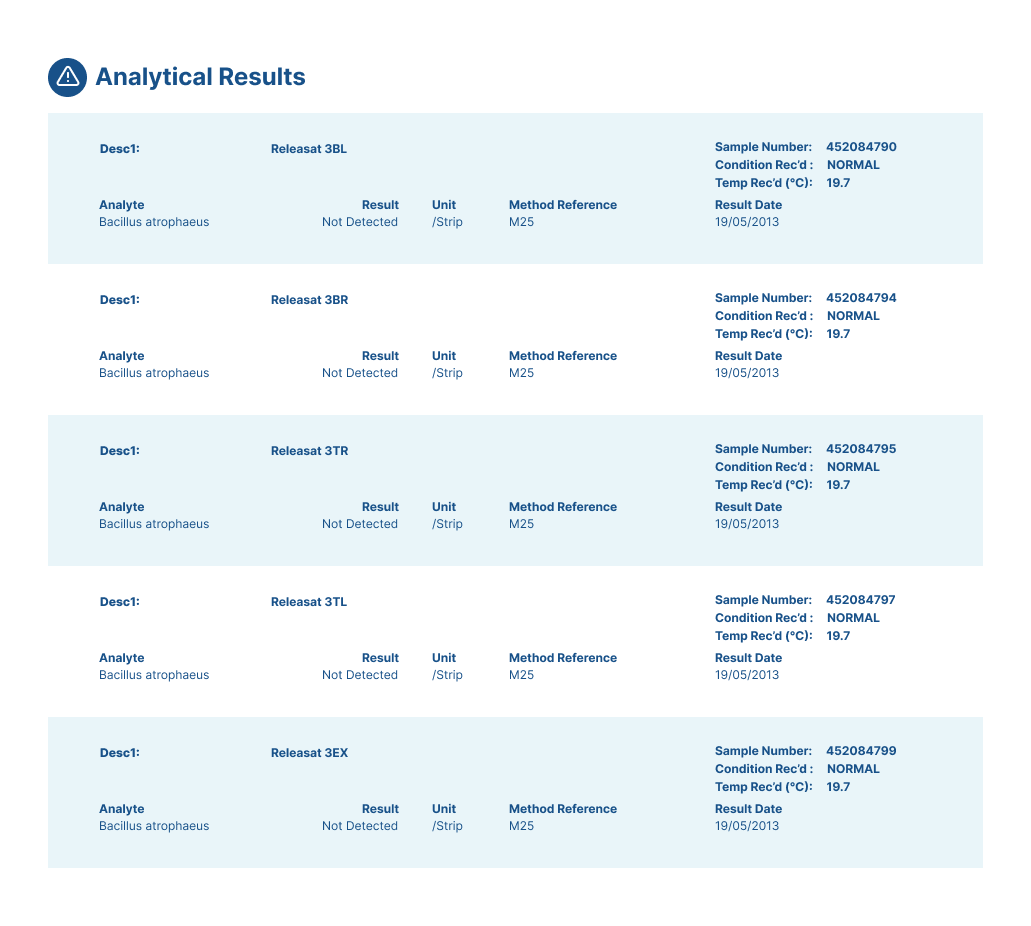
GITASAN ACCREDITATION
HEPA FILTER TESTING
/
FUME CUPBOARD TESTING
/
BIOLOGICAL SAFETY CABINETS
/
CLEAN WORK STATIONS
/
CYTOTOXIC DRUG SAFETY CABINET TESTING
/
CLEANROOM VALIDATION
/
HEPA FILTER TESTING / FUME CUPBOARD TESTING / BIOLOGICAL SAFETY CABINETS / CLEAN WORK STATIONS / CYTOTOXIC DRUG SAFETY CABINET TESTING / CLEANROOM VALIDATION /
Gas Infusion Technology And Sanitation.
GITASAN is a patented decontamination process using fixed quantities of stabilised chlorine dioxide and a proprietary activator (Rainbow DRA2) to generate a predictable release of gas for biological decontamination.
It is designed to eliminate pathogens such as bacteria, viruses, and moulds from surfaces and in the air, leaving no harmful residues.
Biological Decontamination
The GITASAN method is compliant with NSF/ANSI 49-2008 microbiological decontamination guidance (Section G.7) and offers a fast, reliable solution for on-site decontamination in pharmaceutical, healthcare, and research environments.
Trust AG&G Services for critical contamination control testing that protects patients, staff, and operational integrity.
What makes us different
/01
FORENSIC DOCUMENTATION
Every test report is audit-ready, with calibrated equipment, references and detailed analysis.
/02
STRATEGIC INSIGHTS
We identify optimisation opportunities and recommend preventative measures before issues arise.
/03
QUALITY GUARANTEE
Replacement parts always meet or exceed original specifications.
/04
CUSTOM PROTECTION PLANS
Maintenance programs tailored to your operations and risk profile.
/05
TRIPLE-CERTIFIED EXCELLENCE
Three NATA signatories ensure consistent, reliable service delivery.
/06
CUSTOMER-FIRST PHILOSOPHY
Responsive service and a focus on ensuring your needs meet evolving contamination control standards.
While others offer testing services, we deliver compliance assurance. Your controlled environments are too critical to trust to anyone else.